Background: Venetoclax (Ven) in combination with hypomethylating agents (HMAs) is approved in the United States (US) for treatment in patients (pts) with newly diagnosed (ND) acute myeloid leukemia (AML) who are ≥75 years old or unfit for intensive chemotherapy. In a Phase Ib open-label trial (NCT02203773) and the Phase III VIALE-A trial (NCT02993523), a total of 33 pts with ND AML who were treated with Ven+HMAs underwent a stem cell transplant (SCT). The observed 12-month overall survival (OS) was 74.3% post-SCT, and the median OS was 29.9 months for pts who were in remission at the time of SCT (Pratz et al. EHA 2023). This study aims to evaluate the treatment patterns and outcomes of pts with ND AML treated with Ven+HMAs who underwent SCT post-remission from routine clinical practices in the US.
Methods: This was a retrospective cohort study using the Flatiron Health electronic health record-derived, US nationwide, de-identified database. Pts aged ≥18 years with ND AML who initiated first-line Ven+HMAs treatment within 30 days from AML diagnosis (Dx) between July 1, 2018, and November 30, 2022, with ≥2 visits recorded within 3 months of AML Dx, and ≥3 months of potential data available before the study end date (February 28, 2023) were included. Pts with acute promyelocytic leukemia, t(9;22), treated with HMAs before AML Dx and pts who received Ven treatment more than 14 days prior to AML Dx were excluded. Descriptive analysis on patient characteristics was conducted. The proportion of pts who achieved real-world response (defined as <5% bone marrow [BM] blast), median time from Ven+HMAs treatment initiation to last administration (TTLA), and median time from Ven+HMAs initiation to SCT were reported. For pts who received SCT post-remission, 100-day real-world OS (rwOS), 12-month rwOS, and 15-month rwOS were evaluated using Kaplan-Meier analyses. The starting point of Kaplan-Meier analyses was SCT, and pts were censored at the last activity date.
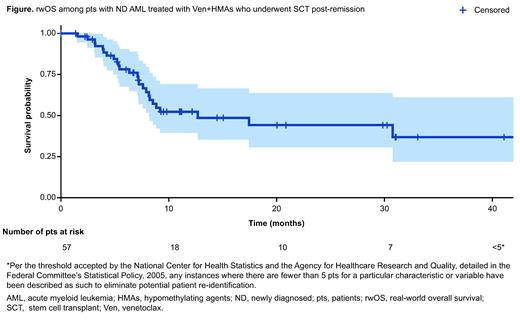
Results: Overall, 988 pts with ND AML treated with Ven+HMAs met the study criteria. A total of 51% (n=503/988) of pts achieved real-world response, of which 11% (n=57/503) subsequently underwent SCT post-remission. In pts with SCT post-remission (n=57) vs pts without SCT post-remission (n=446), the median age was 67 years vs 76 years, 30% (n=17/57) vs 30% (n=134/446) had secondary AML, and 60% (n=34/57) vs 24% (n=107/446) were treated at academic centers, respectively. Excluding pts with unknown cytogenetic and molecular data, the European LeukemiaNet (ELN) 2017 risk in pts with vs without SCT post-remission was favorable for 16% (n=7/43) vs 21% (n=76/355), intermediate for 33% (n=14/43) vs 26% (n=93/355), and adverse for 51% (n=22/43) vs 52% (n=186/355), respectively. Among pts with available molecular testing, FLT3 mutation in pts with SCT vs pts without SCT post-remission was 19% (n=5/26) vs 20% (n=48/245); IDH1/2 mutation was 33% (n=9/27) vs 35% (n=95/275); and TP53 mutation was 47% (n=9/19) vs 28% (n=54/192), respectively. Of the 57 pts who underwent SCT post-remission, the median (interquartile range [IQR]) TTLA on Ven+HMAs was 4.0 (3.0-5.3) months and the median (IQR) time from Ven+HMAs initiation to SCT was 5.4 (4.4-6.8) months. At a median follow-up of 8 months from SCT post-remission, the 100-day rwOS was 92%, 12-month rwOS was 52%, 15-month rwOS was 49%, and the median rwOS was 13.0 (95% confidence interval: 8.2-not reached) months ( Figure).
Conclusions: In this study, similar to findings from clinical trials, we observed a subset of pts with ND AML treated with Ven+HMAs underwent SCT post-remission in routine clinical setting. Compared with pts who did not undergo SCT post-remission, pts who underwent SCT post-remission were younger, and more of these pts received care in academic centers. Of the Ven+HMAs-treated pts who underwent SCT post-remission, more pts treated in the routine clinical setting had the poor prognostic TP53 mutation than pts enrolled in the aforementioned clinical trials. In this study, most pts remained alive 100 days after SCT. The rwOS results observed in this study suggest that Ven+HMAs post-remission is a potential bridge to SCT in pts who are ineligible for intensive chemotherapy.
Disclosures
Bachiashvili:University of Alabama at Birmingham: Current Employment. Ma:Genentech, Inc.: Current Employment, Current equity holder in private company. Patel:GlaxoSmithKline (via University of North Carolina - Chapel Hill), Regeneron: Ended employment in the past 24 months; Genentech, Inc.: Current Employment; Genentech, Inc. (F. Hoffmann-La Roche Ltd): Current equity holder in publicly-traded company. Wang:F. Hoffmann-La Roche Ltd (stock and options as an employee): Current equity holder in publicly-traded company; Genentech, Inc.: Current Employment. Yellow-Duke:Genentech, Inc.: Current Employment, Current equity holder in private company, Current equity holder in publicly-traded company, Current holder of stock options in a privately-held company. Montez:Genentech, Inc./F. Hoffmann-La Roche Ltd: Current Employment; F. Hoffmann-La Roche Ltd: Current equity holder in publicly-traded company. Bui:AbbVie Inc: Current Employment, Current holder of stock options in a privately-held company. Worth:Blueprint, CTI BioPharma: Consultancy; University of Alabama at Birmingham: Current Employment. Vachhani:Incyte, CTI BioPharma Corp, Blueprint Medicines: Speakers Bureau; Abbvie, Amgen, Blueprint Medicines, Cogent Biosciences, Incyte, CTI BioPharma Corp, Daiichi Sankyo, GlaxoSmith Kline, Karyopharm, Novartis, Pfizer, Genentech, Inc., Servier, Stemline, MorphoSys, LAVA therapeutics: Honoraria.